The elastic collisions, where the principal quantum number of the
exotic atom is not changed, play two important roles in the atomic
cascade. First, the Stark mixing is nothing but the elastic scattering
as long as only the rates of transitions between the ![]() -sublevels of
the same
-sublevels of
the same ![]() are concerned. Second, the energy losses in elastic
collisions lead to the deceleration of the epithermal exotic atoms.
are concerned. Second, the energy losses in elastic
collisions lead to the deceleration of the epithermal exotic atoms.
The basic features of the elastic scattering can be explained in the
semiclassical approximation [23]. The motion of
the ![]() atom with the parabolic quantum numbers
atom with the parabolic quantum numbers ![]() in the electric field of a hydrogen atom is described by the
effective potential3
in the electric field of a hydrogen atom is described by the
effective potential3
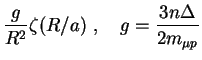 |
(6) |
A semiclassical model of the Stark mixing was developed in [1] and used with some variations and refinements in [6,7,8,10]. In this model, the exotic atom moves along a straight line trajectory with a constant velocity through the electric field of a hydrogen atom, and the Stark transitions induced by this electric field are calculated. Instead of straight line trajectories the classical trajectories can be used as in [28]. The characteristic scale of the Stark mixing cross section is determined by the electron screening of the proton electric field, i.e. by the size of the hydrogen atom which makes Stark mixing the fastest collision process in the atomic cascade, see Figs. 2.
Recently the problem of the elastic scattering of exotic atoms in
excited states was treated in a quantum mechanical framework using the
adiabatic expansion [9,11,15], the calculation being done
in a single-channel adiabatic approximation. The results show
fair agreement with the semiclassical calculations
[14] for the collision energies ![]() eV. A detailed discussion
of the Stark collisions, including the relative role of transitions with
different
eV. A detailed discussion
of the Stark collisions, including the relative role of transitions with
different
![]() can be found in [11]. It would be
desirable to extend the quantum mechanical model of the elastic
collisions by including the effects of the shift and width of the
can be found in [11]. It would be
desirable to extend the quantum mechanical model of the elastic
collisions by including the effects of the shift and width of the
![]() states as well as the coupling between different adiabatic terms.
states as well as the coupling between different adiabatic terms.
The elastic scattering leads to the deceleration of exotic atoms
if the kinetic energy is larger than the target temperature.
The effective deceleration rate for the ![]() collisions is defined
by the transport cross section:
collisions is defined
by the transport cross section:
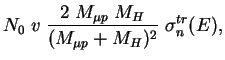 |
(9) | ||
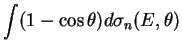 |
(10) |
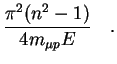 |
(11) |