The intensities of the X-ray yields in the ![]() and
and ![]() atoms calculated with the Monte Carlo code [62,56] are shown in Table
3. The cascade model used is in good agreement with the experimental data on
the
atoms calculated with the Monte Carlo code [62,56] are shown in Table
3. The cascade model used is in good agreement with the experimental data on
the ![]() X-ray yields [63] and in fair agreement with
X-ray yields [63] and in fair agreement with ![]() data [56,64].
data [56,64].
| |||||||||||||||||||||||||||||||||||
Important for the proposed experiment is the fact that the muonic
![]() and
and
![]() transitions are about an order
of magnitude stronger than the corresponding pionic transitions. This compensates
the lower stop efficiency for muons. An extension of the measurement to the
transitions are about an order
of magnitude stronger than the corresponding pionic transitions. This compensates
the lower stop efficiency for muons. An extension of the measurement to the
![]() transition can also be considered; the intensity, however,
decreases significantly for higher pressures. In addition the Bragg angles are
becoming small and make this transition unfavorable. Rate estimates based on
the yields of Table 3 are given in chapter 3.6.
transition can also be considered; the intensity, however,
decreases significantly for higher pressures. In addition the Bragg angles are
becoming small and make this transition unfavorable. Rate estimates based on
the yields of Table 3 are given in chapter 3.6.
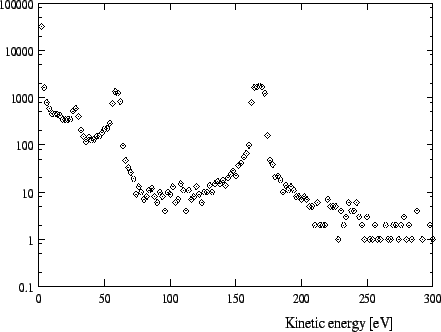
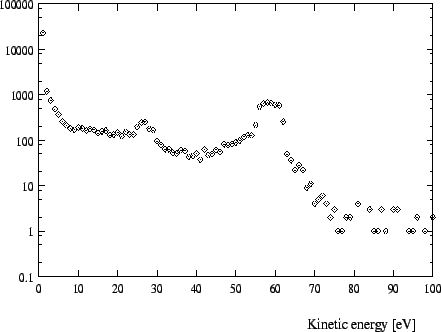
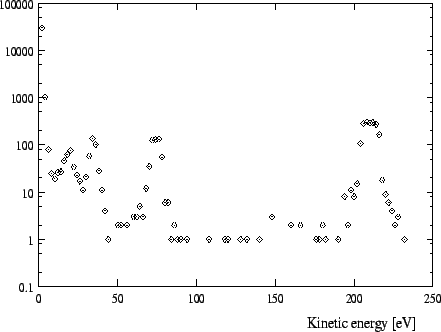
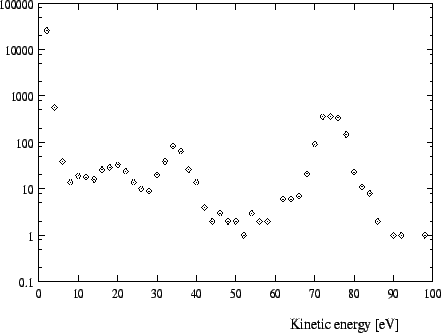
In Figures 1 -4 calculated distributions of kinetic energies are shown
for a pressure of 15 ![]() for the exotic hydrogen atoms at the instant
of the
for the exotic hydrogen atoms at the instant
of the
![]() and the
and the
![]() transition. (We always
give the values for the pressures at a temperature of 293K.) The influence of
these distributions on the form of the X-ray lines is depicted in Fig. 8-9.
transition. (We always
give the values for the pressures at a temperature of 293K.) The influence of
these distributions on the form of the X-ray lines is depicted in Fig. 8-9.
|
|
|
|